White PaperArticle
NHS Scotland Assessment of LumiraDx SARS-CoV-2 Ab Test
Introduction
NHS Lothian assessed the performance of the LumiraDx SARS-CoV-2 Ab (Antibody) Test during 2020 and 2021. The main aim of the evaluation was to assess the sensitivity and specificity of the LumiraDx SARSCoV- 2 Ab Test, whilst also investigating any changes in sensitivity from infection as time progresses, and comparing the performance of the test using serum with capillary samples and against neutralising antibody titres.
The LumiraDx SARS-CoV-2 Ab Test is a microfluidic immunofluorescence assay for qualitative detection of total antibodies to SARS-CoV-2 in human whole blood (capillary fingerstick or venous), plasma, or serum for indication of recent or prior infection. Used with the LumiraDx Platform, the test delivers rapid results in approximately 11 minutes at the point of care. The LumiraDx SARS-CoV-2 Ab Test is designed to be used in community care settings to identify individuals with an adaptive immune response to COVID-19, indicating recent or prior infection.
Methods
There were four evaluation panels used in the assessment:
- The sensitivity and specificity of the LumiraDx SARS-CoV-2 Ab Test was assessed using samples from RTPCR positive individuals and pre-pandemic stored sera, respectively.
- Longitudinal samples were collected from individuals with RT-PCR confirmed SARS-CoV-2 infection who were not hospitalised during the course of their illness. These samples are considered to be representative of the majority of SARS-CoV-2 infected individuals.
- The sensitivity of the LumiraDx Test using capillary blood samples was evaluated alongside a paired serum sample to assess their comparability.
- Serum samples were tested for neutralising antibody (NT50) titres based on a pseudotyped lentivirus assay1. A value between 50 and 160 was considered detectable, and values >160 considered a significant antibody result which may confer protection against reinfection2. These neutralising antibody titres were compared to the results obtained from serum samples on the LumiraDx Test.
Results
The tests were performed according to the LumiraDx SARS-CoV-2 Ab Test Product Insert3.
Table 1: Sensitivity and specificity
| Sensitivity (all time points) | Sensitivity (>20 days post infection) | Specificity | |
|---|---|---|---|
| Number of samples | 262 | 200 | 201 |
| % | 94.3% | 98.1% | 99.5% |
The sensitivity panel that was used in this evaluation were all serum samples taken from PCR-positive individuals including sera from hospitalized patients and non-hospitalized COVID-19 convalescents.
The specificity panel included samples from blood transfusions donors, individuals recently infected with other seasonal coronaviruses, respiratory pathogens, Epstein-Barr Virus (EBV) Ab positive, Cytomegalovirus (CMV) Ab positive, antenatal samples and Rheumatoid factor. All samples were collected prior to the pandemic.
Sensitivity with time
LumiraDx sensitivity did not change as time from symptom onset increased, with a sensitivity of 97.6% (n=127) at 20-79 days post PCR, 97.7% (n=43) at 80-139 days post PCR and 100% (n=30) >140 days.
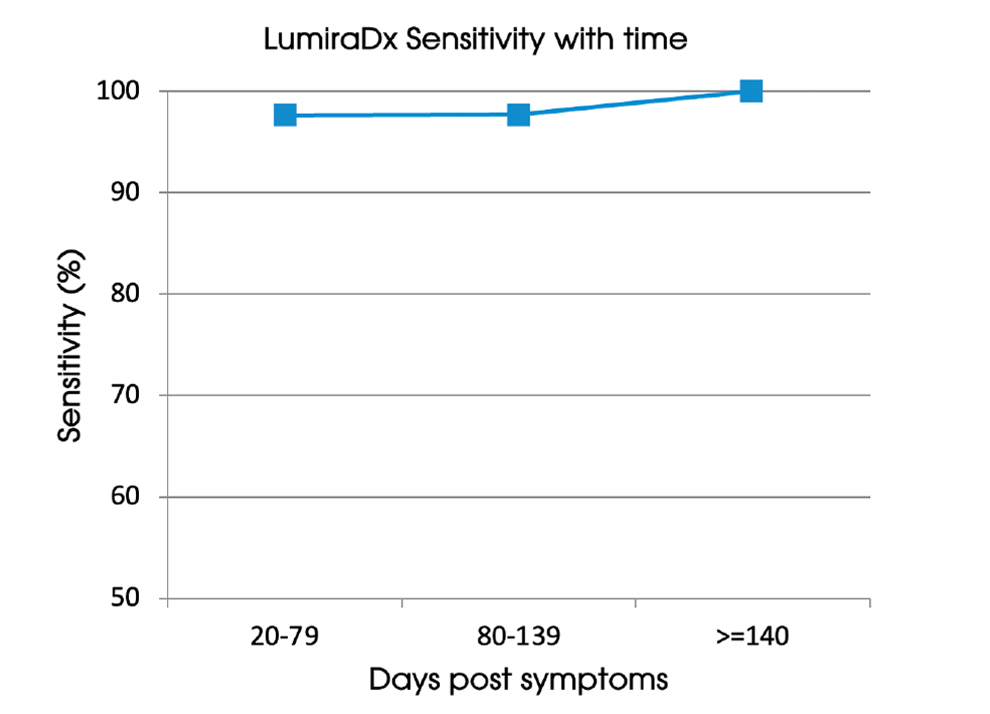
Figure 1: LumiraDx sensitivity with time since symptom onset
Serum Vs capillary performance
The paired capillary and serum LumiraDx results were compared in 50 participants at NHS Tayside (Table 2). Tests were carried out by Research Clinic Medical staff at NHS Tayside testing, namely the Tayside Point-of- Care Test Co-ordinator.
Amongst the NHS Tayside participants, 14 had previously had a PCR confirmed COVID-19 infection and the other 36 were included due to having been previously antibody positive using the Siemens Total COVID antibody assay.
Table 2 – Comparison of performance on paired serum and capillary samples at NHS Tayside
| LumiraDx positive (capillary blood) | LumiraDx positive (Serum) | |
|---|---|---|
| NHS Tayside PCR positive (n=14) | 14 (100%) | 14 (100%) |
| NHS Tayside Siemens Ab positive (n= 36)* | 29 (81%) | 32 (89%) |
*Seven out of thirty-six Siemens Ab positive individuals tested negative with LumiraDx. All were invited back to provide another sample to evaluate Siemens Ab levels. Of these, five individuals provided another sample, with two out of five testing negative with the Siemens reference method and the other three giving positive results.
Comparison of LumiraDx Test Performance with Neutralising Ab Titres
The performance of the LumiraDx Test on serum was compared to neutralising antibody titres on 198 samples. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for two NT50 thresholds (NT50 ≥ 50 i.e. detectable, and NT50 ≥ 160, i.e. correlated with protection in some studies2). The results show (Table 3) that all patients with significant neutralising titres (>160) showed a positive serum LumiraDx result. However, of the 28 serum samples which showed undetectable (<50) neutralising titres, 26 showed a positive antibody LumiraDx result indicating that a positive result with LumiraDx is not a very good proxy for the presence of protective neutralising antibodies. A negative result however does have a high negative predictive value and could be used to determine the absence of neutralising antibodies.
Table 3: Comparison of neutralising antibodies titres to serum result with the LumiraDx SARS-CoV-2 Ab Testresult (n=198).
| LumiraDx NT50 ≥50 | LumiraDx NT50 ≥160 | |
|---|---|---|
| TP | 168 | 112 |
| FN | 2 | 0 |
| FP | 26 | 82 |
| TN | 2 | 4 |
| Sens | 98.8 | 100.0 |
| Spec | 7.1 | 4.7 |
| PPV | 86.6 | 57.7 |
| NPV | 50.0 | 100.0 |
TP = true positive, FP= false positive, TN = true negative, FN= false negative
Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of the LumiraDx assay for the detection of neutralising antibodies. It is noted that the PPV and NPV are affected by the prevalence of neutralising antibodies within the cohort of individuals being tested and these values are therefore specific to this cohort.
Summary
In this evaluation from NHS Lothian, the LumiraDx SARS-CoV-2 Ab Test was demonstrated to be highly sensitive, remaining highly sensitive as time from infection increases. This indicates that this test is well suited to sero-surveillance studies where the primary goal is to identify individuals who have previously been infected.The performance on capillary blood versus serum samples were very good in the evaluation at NHS Tayside.
The neutralising antibody data indicates that the LumiraDx SARS-CoV-2 Ab Test detects antibodies in some individuals in the absence of significant neutralising antibody levels and it is therefore recommended that a positive result with this test kit is not assumed to indicate the presence of neutralising antibodies i.e. a positive result should not be assumed to confer significant immunity. However, a negative result could be used to indicate the absence of neutralising antibodies.
References
- Schmidt et. al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. JEM 2020 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7372514
- Addetia et. al. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. Journal of Clinical Microbiology Oct 2020, 58 (11) e02107-20; DOI: 10.1128/JCM.02107-2
- LumiraDx SARS-CoV-2 Ab Test Product Insert can be found here
The LumiraDx SARS-CoV-2 Antibody Test has achieved CE Mark.
In the U.S.A the LumiraDx SARS-CoV-2 Ab Test has not been FDA cleared or approved but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories. This product has been authorized for use with a test authorized only for detecting the presence of total antibodies to SARS- CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
LumiraDx UK Ltd: Unit 1, Block 5, Dumyat Business Park, Bond Street, Alloa, FK10 2PB, UK
Copyright ©2021 LumiraDx UK LTD. All rights reserved worldwide. Content should be used for the use of the LumiraDx products only and in line with instructions provided. You may not, except with our express written permission, distribute or commercially exploit the content. Nor may you transmit it or store it in any other form of electronic retrieval system other than for the purpose of use of the LumiraDx Instrument or LumiraDx Test Strips. Information provided is subject to change without notice.
LumiraDx and Flame logo are trademarks of LumiraDx International LTD. Full details of these and other registrations of LumiraDx can be found at lumiradx.com/IP. All other trademarksare the property of their respective owners.