SARS-CoV-2 Ag Test
an easy to use, fast microfluidic immunofluorescence assay designed to rapidly quantify nucleocapsid protein antigen in nasal and nasopharyngeal swab specimens. Used with the LumiraDx Platform, the LumiraDx SARS-CoV-2 Ag test provides actionable, lab-comparable results in just 12 minutes.
Qualitative SARS-CoV-2 Ag test
The LumiraDx SARS-CoV-2 Ag test is an automated rapid microfluidic immunofluorescence assay for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 Ag directly from nasal swab and nasopharyngeal swab samples collected from individuals suspected of COVID-19 or from asymptomatic individuals.
Near-patient testing
As an automated in vitro diagnostic test, it is made for near-patient testing with the LumiraDx Instrument.
Purpose
The test can be used as an aid in the diagnosis of SARS-CoV-2 infection by detection of SARS‑CoV‑2 antigen.
Benefits

Verify COVID-19 quickly
Identify potentially infected and contagious spreaders quickly. Actionable and lab-comparable results for patients suspected of COVID-19.
- Sample types: nasal and nasopharyngeal swab
- Nasal swabs (symptomatic)
- 97.6% PPA versus RT-PCR
- 96.6% NPA versus RT-PCR
- Time to result: 12 minutes
- Storage at room temperature (2°C to 30°C)

Workflow
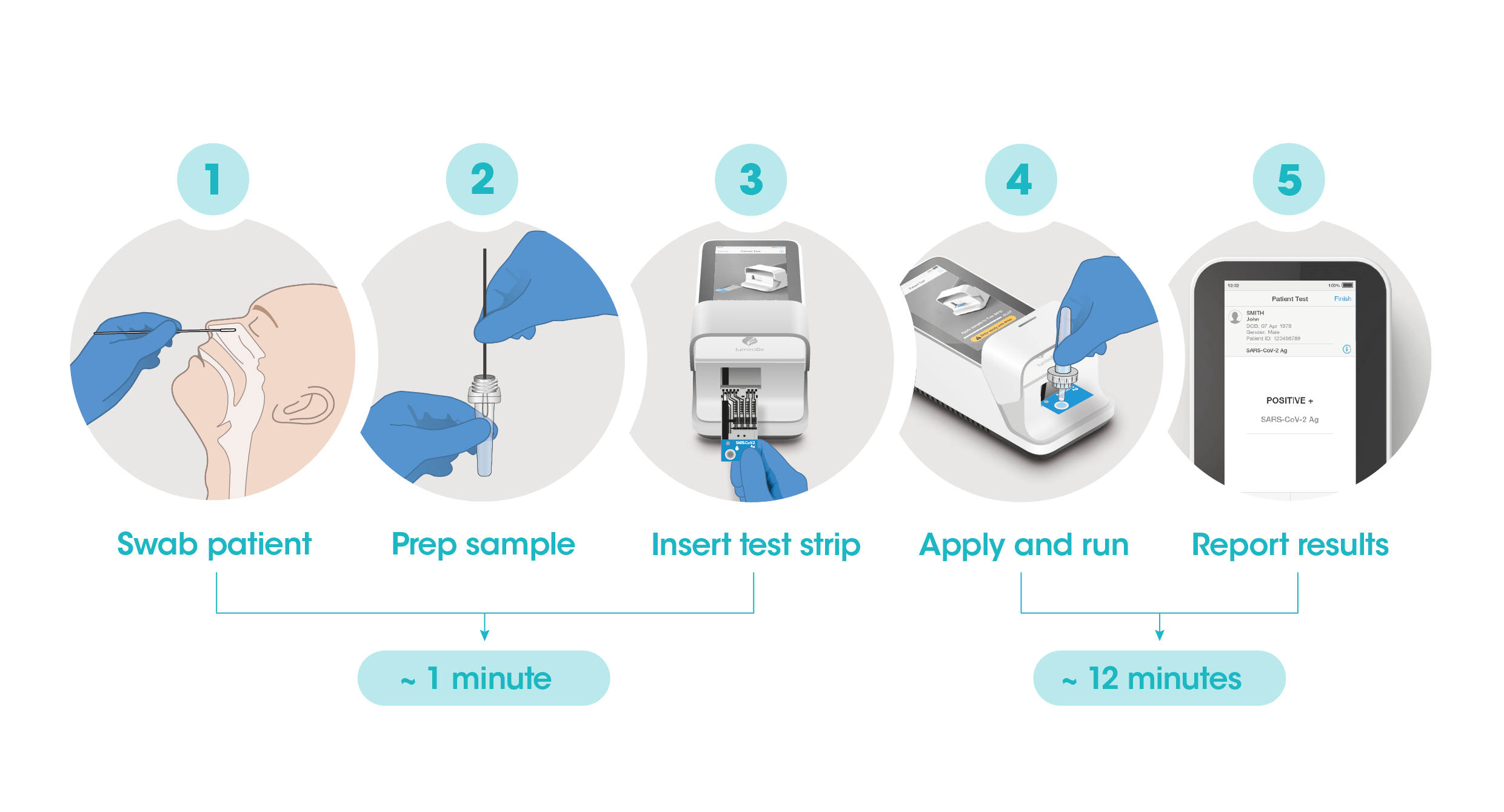
The workflow process is comprised of a simple sample prep along with step-by-step guidance of the Instrument to report a patient result in 12 minutes from sample application.
The Instrument and test strips are integrated with several quality control checks to ensure the Instrument and test are functioning correctly for every test run.
HOW TO USE VIDEOTest Performance
Clinical performance
In clinical studies, the LumiraDx SARS-CoV-2 antigen test demonstrated 97.6% positive agreement versus RT-PCR in patients tested within 12 days of the onset of symptoms, to enable the physician to verify infection quickly, begin proper treatment and to initiate isolation precautions helping prevent further spread of infection.
Analytical performance
The limit of detection was found to be 32 TCID50/mL using a starting concentration of 2.8 x 105 TCID50/mL with 20/20 positive results.
Cross reactivity
The LumiraDx SARS-CoV-2 Ag test was found not to cross-react with a panel of organisms and viruses including several human coronaviruses. See LumiraDx SARS-CoV-2 Ag test Product Insert for full details.
Product Documentation
SARS-CoV-2 Ag Test:
SARS-CoV-2 Ag Test

