SARS-CoV-2 & RSV Test
a rapid microfluidic immunofluorescence assay designed to optimize patient and clinical flow. Used with the LumiraDx Platform, the LumiraDx SARS-CoV-2 & RSV test provides actionable and lab-comparable results in 12 minutes for patients suspected of RSV and/or COVID-19.
Qualitative SARS-CoV-2 & RSV test
The LumiraDx SARS-CoV-2 & RSV test is an automated rapid microfluidic immunofluorescence assay for the qualitative detection and differentiation of SARS-CoV-2 and RSV viral antigens directly from anterior nasal swab samples.
Near-patient testing
As an automated in vitro diagnostic test, it is made for near-patient testing with the LumiraDx Instrument.
Purpose
The test can be used as an aid in the differential diagnosis of SARS-CoV-2 and RSV.
Benefits

Actionable results in minutes
Verify potential infection and help guide infection control measures quickly:
- Optimize patient and clinical flow - 1 sample, 2 results, 12 minutes
- Test: RSV and COVID-19
- Sample type: nasal swab
- Prepared sample can also be used with SARS-CoV-2 & Flu A/B*
- SARS-CoV-2 - PPA** 98.3%
- RSV - PPA*** 93.5%
- Time to result: 12 minutes
- Storage at room temperature (2°C to 30°C)
*Swab must be validated for use with both tests - refer to swab technical bulletins
**Ct <33 *** Ct <30 Refer to SARS-CoV-2 & RSV Product Insert for full clinical data

Workflow
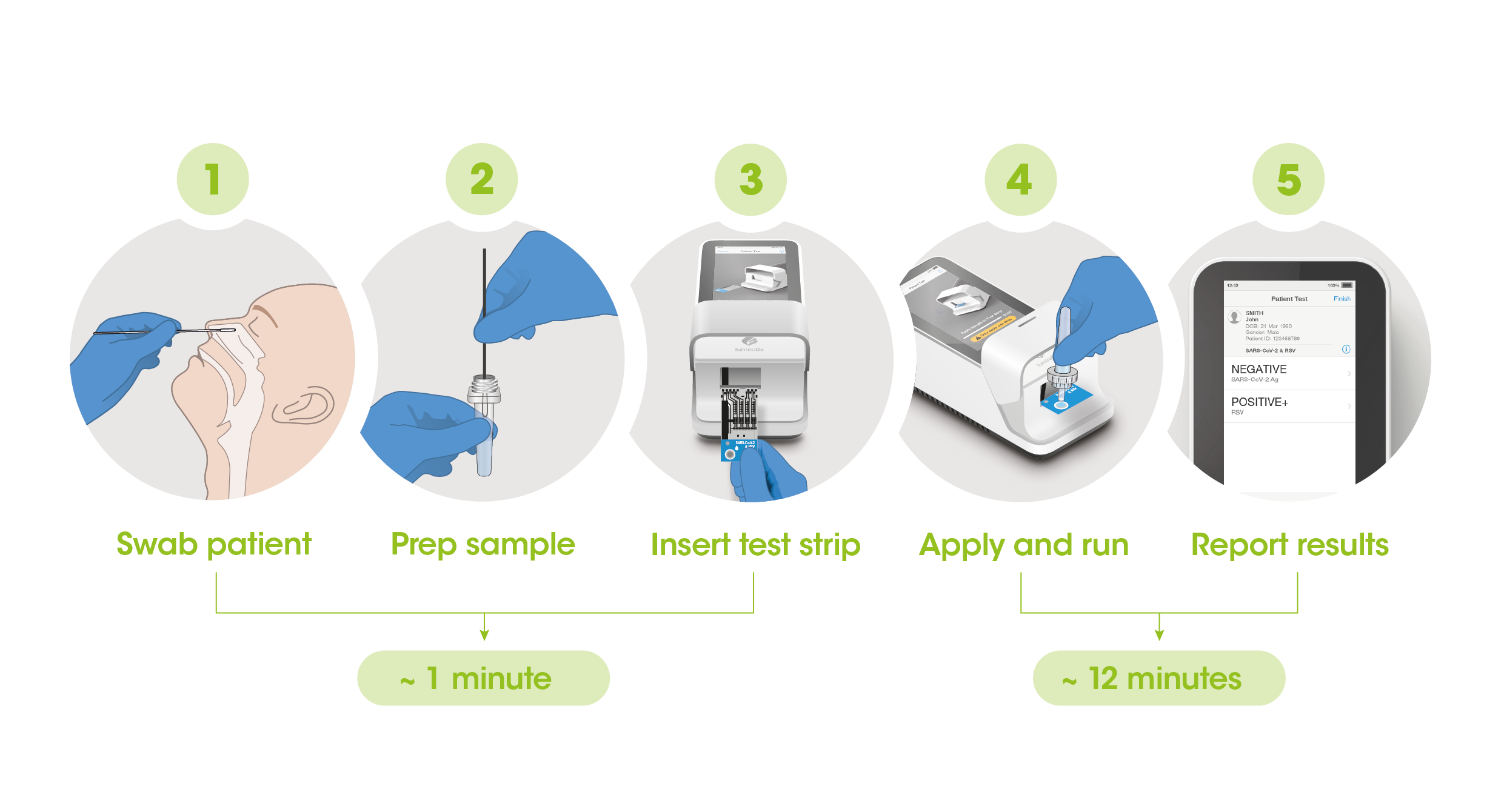
The workflow process is comprised of a simple sample prep along with step-by-step guidance of the Instrument to report a patient result in 12 minutes from sample application.
The Instrument and test strips are integrated with several quality control checks to ensure the Instrument and test are functioning correctly for every test run.
HOW TO USE VIDEOTest Performance
Clinical performance
In clinical studies, the LumiraDx SARS-CoV-2 & RSV test demonstrated up to 98.3% and 93.5% positive agreement versus RT-PCR for detection of SARS-CoV-2 and RSV respectively.
Analytical performance
The final limit of detection of the LumiraDx SARS-CoV-2 & RSV test was determined to be the lowest concentration resulting in positive detection of at least 95% of replicates. Click above for the LoD values for different virus materials.
Cross reactivity
SARS-CoV-2 & RSV was found not to cross-react with a panel of organisms and viruses including several human coronaviruses. See LumiraDx SARS-CoV-2 & RSV Product Insert for full details.
Product Documentation
SARS-CoV-2 & RSV Test:
SARS-CoV-2 & RSV Test

