D-Dimer
an easy to use, fast microfluidic immunoassay designed to rapidly quantify D-Dimer levels in whole blood and plasma. It is the only quantitative direct fingerstick D-Dimer assay available today*, aiding healthcare professionals to exclude deep vein thrombosis (DVT) and pulmonary embolism (PE) in symptomatic patients with confidence - all in only 6 minutes.**
*As stated at time of publication - 04/03/2024
**In conjunction with a clinical pre-test probability assessment model
Quantitative D-Dimer
The LumiraDx D-Dimer test is an in vitro diagnostic test for the quantitative determination of D-Dimer in human capillary and venous whole blood and plasma samples (Sodium Citrate).
Near-patient testing
As an automated in vitro diagnostic test, it is made for near-patient testing with the LumiraDx Instrument.
Purpose
The test can be used in conjunction with a clinical pretest probability assessment model to exclude DVT and PE disease for symptomatic patients.
Benefits

Improving efficiency
Improve efficiency in primary and secondary care settings by offering a rapid assessment of patients presenting with symptoms of deep vein thrombosis (DVT) and pulmonary embolism (PE):
- Clinical cut-off: 500 μg/L Fibrinogen Equivalent Units (FEU), 0.500 mg/L FEU, 500 ng/mL FEU, 0.500 μg/mL FEU
- Sample types: direct fingerstick (or via lithium heparin transfer tube), venous (sodium citrate) or plasma (sodium citrate)
- Sample size: 15μL
- Hematocrit determination for whole blood sample to ensure 20 - 55% range
- Reportable range: 190 - 4000 g /L FEU, 0.190 - 4.000 mg/L FEU, 190 - 4000 ng/mL FEU, 0.190 - 4.000 μg/mL FEU
- Time to result: 6 minutes
- Storage at room temperature (2°C to 30°C)

Workflow
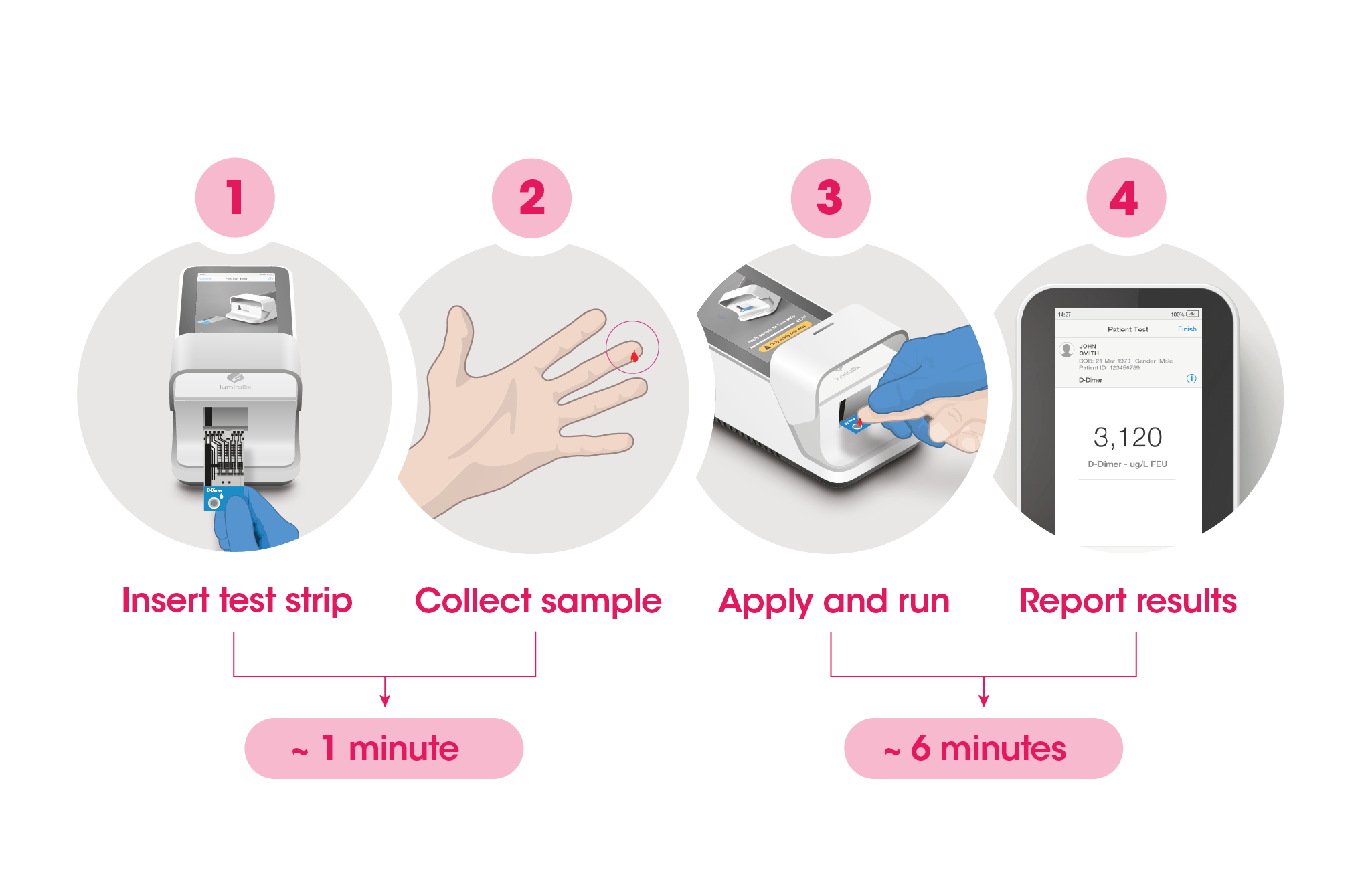
Test results in 6 minutes
The workflow process is comprised of a fingerstick sample collection, followed by step-by-step guidance of the Instrument to report a patient result in 6 minutes from sample application.
The Instrument and test strips are integrated with several quality control checks to ensure the Instrument and test are functioning correctly for every test run.
HOW TO USE VIDEOTest performance
Clinical performance
A prospective clinical study was performed on 585 subjects where capillary blood, venous (blood citrated) and plasma (citrated) samples were collected from patients with VTE symptoms following a Wells score classification. Of those with 'unlikely' PTP categorization 100% sensitivity and 100% NPV was demonstrated across all three sample types. Click above for further details.
Method comparison
The method comparison was performed using plasma samples from patients, n=327, range= 60 – 4515 µg/L FEU, (0.06 – 4.515 mg/L FEU), (60 – 4515 ng/mL FEU), (0.06 – 4.515 µg/mL FEU). 1767 D-Dimer measurements with the LumiraDx D-Dimer test and the VIDAS Exclusion II D-Dimer assay was completed giving a slope of 1.02, intercept of 21 and r value of 0.92.
Precision
A precision study was carried out in citrated venous plasma with 3 levels of D-Dimer, each was tested in 2 runs of 2 replicates per day, for twenty days. The precision ranged from 9.7% CV to 11.1% CV across the three levels. Click above for further details.
Product Documentation
D-Dimer Test:
D-Dimer Test

