CRP Test
an easy to use, fast microfluidic immunoassay designed to rapidly quantify CRP levels in whole blood and plasma. Used with the LumiraDx Platform, the LumiraDx CRP test delivers rapid, quantitative results in 4 minutes at the point of care.
Quantitative CRP test
The LumiraDx CRP test is an in vitro diagnostic test for the quantitative determination of C-Reactive Protein in human whole blood (capillary fingerstick and venous) and plasma samples.
Near-patient testing
As an automated in vitro diagnostic test, it is made for near-patient testing with the LumiraDx Instrument.
Purpose
The measurement of CRP provides information for the detection and evaluation of infection, tissue injury, inflammation disorders and associated disease.
Benefits

Rapidly identify CRP levels in just 4 minutes
A quantitative, direct fingerstick, determination of C-Reactive Protein. Help identify infection, tissue injury and inflammation disorders:
- Sample types: fingerstick (or via lithium heparin transfer tube), venous (lithium heparin), or plasma (lithium heparin)
- Sample size: 20 μL
- Hematocrit determination for whole blood sample to ensure 15 - 55% range
- Reportable range 5 - 250 mg/L
- Time to result: 4 minutes
- Storage at room temperature (2°C to 30°C)

Workflow
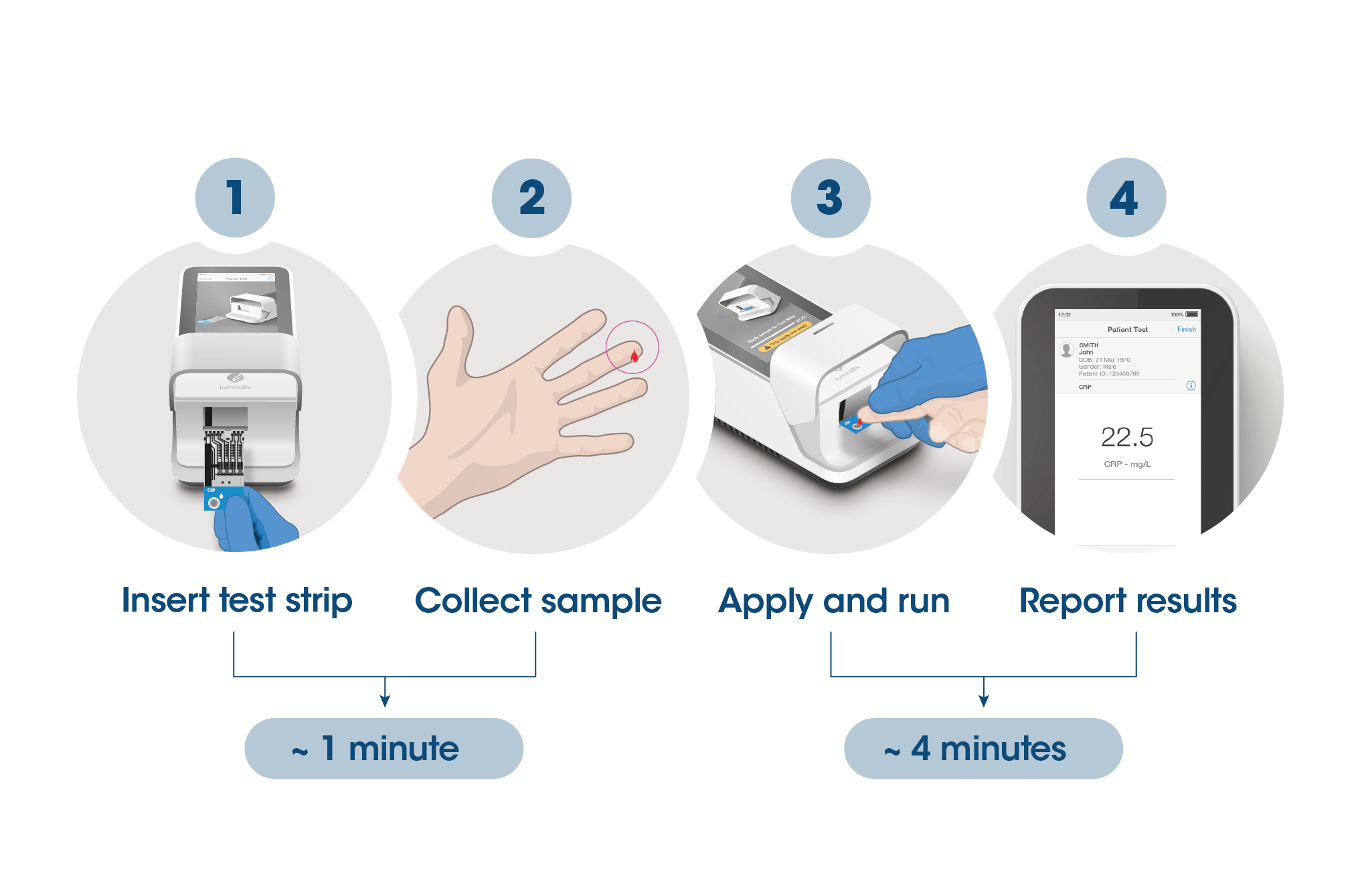
Test results in 4 minutes
The workflow process is comprised of a fingerstick sample collection, followed by step-by-step guidance of the Instrument to report a patient result in 4 minutes from sample application.
The Instrument and test strips are integrated with several quality control checks to ensure the Instrument and test are functioning correctly for every test run.
HOW TO USE VIDEOTest Performance
Method comparison
A comparison of 205 CRP measurements with the LumiraDx CRP test and the RCRP Flex® assay on the Siemens Dimension® Xpand® Plus Integrated Chemistry System was completed and analysed by Passing Bablok regression giving a slope of 1.00, intercept of -0.48 and an r value of 0.99. Click above for more details.
Test precision
A precision study was carried out in heparinised venous plasma on a protocol based on CLSI EP5-A3. The study was carried out at 3 concentrations of CRP, each was tested in 1 run of 5 replicates per day, for five days across 3 sites. The results of the precision study were 7.4%, 6.0% and 5.6% across the three levels. Click above for more details.
Product Documentation
CRP Test:
CRP Test

